Contents
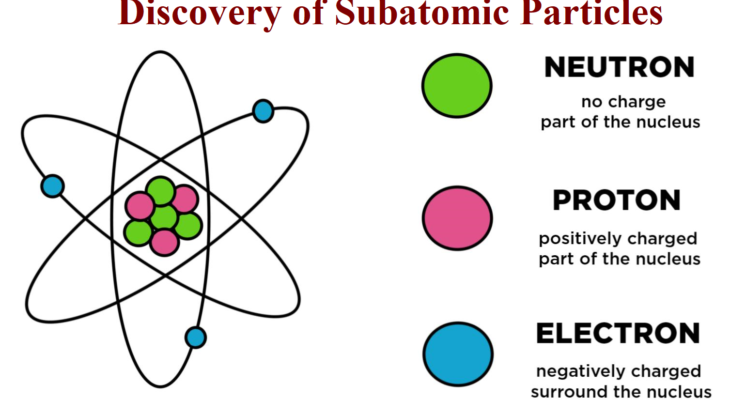
Discovering Subatomic Particles: Proton, Neutron and Electrons
Dalton’s theory was established in 1803. Dalton theory states that the atom is the smallest indivisible constituent of all matter. However, towards the end of the 19th century and in the 20th century, Dalton theory was disproved. It was observed that atoms could be divided into even small particles. These divided small particles were called subatomic particles, also named elementary particles; the important subatomic particles are Protons, Neutrons and Electrons.
Let’s learn about the discovery of Neutron, protons and electrons in its article.
Proton
Protons are positively charged particles. A Proton has a positive electric charge of 1e (symbol p or p ), primary charge and a mass are less than neutron particles. In the nucleus of an atom, the total number of protons is nothing but the Atomic Number (Z) of an atom.
It is represented as;
No. of Protons = Atomic Number
For Example, the atomic number of the Carbon (C) atom is 6.
Hence, the nucleus of the Carbon atom contains 6 protons.
Proton Discovery
- Rutherford’s Gold Foil Experiment discovered protons in 1909. Rutherford on a very thin gold foil allowed to pass through alpha radiation and then observed the scattered alpha radiation on-screen and observations for the experiment as follows:
- More alpha radiation passed through the foil without deviating.
- Less alpha radiation got deviated at a small angle.
- Very little alpha radiation was reflected.
Based on the above experimental observations, he stated the following:
- The atom’s mass and its positive charge are combined in a small core, called the nucleus.
- The volume of an atom is empty space.
- The number of negatively charged electrons around the nucleus equals that of the number of positively charged protons in the nucleus.
Properties of Protons:
- Protons travel in narrow lines and can produce a shadow of the object placed in their radiation travel path.
- The radiation constituting anode rays contains mass and has kinetic energy.
- Electric and magnetic fields deflect these radiations.
- The weight of the proton is found to be 1.672 x 10-24 g.
- The proton charge is found to be 1.602 x 10-19 C.
- The proton volume is 4/3 πr3 (1.5 x 10-38 cm3)
Neutrons
James Chadwick discovered that the nucleus had a new uncharged particle; Chadwick named it neutron in 1932. It is a subatomic particle (symbol n or n0), which has a neutral charge, and its mass is greater than a proton. The Centre of the atom is called the Nuclei; it has Protons and neutrons. Both Protons and Neutrons are together called “nucleons”.
Neutrons Discovery
Beryllium was heavily bombarded with high energy alpha radiation; he discovered the presence of a new particle called a neutron, which has less charge and similar mass than the proton. Beryllium undergoes the following reaction when it is bombarded with alpha radiation.
94Be + 42He → [136C]→126C + 10n
(Alpha particle) (Beryllium) (Carbon) (Neutron)
For this discovery, James Chadwick was awarded the Nobel Prize in 1935. The total number of protons and neutrons is the mass number of atoms.
It is represented as;
Mass Number = (No. of Protons) (No. of Neutrons)
Or
No. of Nucleons = Mass Number – Atomic Number
For Example, Neon
Mass number = 20.18
Protons = 10
20.18 = 10 (Number of Neutrons)
Number of Neutrons = 20.18 –10 = 10.18
Properties of Neutrons:
- Neutrons specific charge is zero
- Neutrons are neutral particles that carry no charge
- The mass neutrons are the same as that of protons
- Neutron density is 1.5 x 1014 g/cc.
- Except for hydrogen, all other atoms contain neutrons.
Electron
The Electron has a negative charge (symbol e− or β−) whose electric charge is negative. The electron has a mass that is approximately 1/1836 that of the proton. Electrons surround the nucleus of the atom. There is usually a higher probability of finding an electron closer to the nucleus of an atom. Electrons can abbreviate as e–.
Electrons Discovery
J.J. Thomson discovered electrons in 1897 by using the Cathode Ray Tube experiment; a very high voltage of electricity was passed through a cathode tube containing a noble gas at low pressure. He observed that a new particle was produced from the cathode (negative electrode), which moves towards the anode (positive electrode).
Robert Millikan, through oil drop experiments, found the value of the electronic charge. These new particles were coined as cathode rays. Cathode rays are particles that are negatively charged; these cathode rays are called electrons.
The key characteristics of cathode rays are as follows:
- Electrons are negatively charged particles that are equal in magnitude to protons.
- The mass of an electron is lesser than a proton or neutron.
- Electron carry mass and have kinetic energy
- Electrons travel in a linear path.
- The mass and charge of electrons produced in the cathode ray tube are independent of the gas-filled nature.
It is represented as;
No. of Electrons = Number of Protons = Atomic Number.
For example,
The nucleus of an atom of Neon has 10 protons in it. The balance between protons and electrons is maintained when a Neon atom has 10 electrons.
Properties of Electrons:
- 1 mole of electron charge is 1 faraday or 96450 C
- The electron specific charge (e/m) is 1.76 x 108 coulomb/gram
- An increase in the velocity of electrons reduces the specific charge.
- Electron radius is 10-15 cm.
- Electron density is 2.17 x 1017 g/cc.
Summary: Protons Neutrons and Electron;
| Sl. No. | Properties | Proton | Neutron | Electron |
| 1 | Symbol | p1 or po | n1 or no | e1 or eo |
| 2 | Charge | 1.6×10−19 C | 0 | −1.6×10−19 C |
| 3 | Mass | 1.67 ×10−27 kg | 1.67 ×10−27 kg | 9.11 ×10−31 kg |
| 4 | Location | Inside the nucleus | Inside the nucleus | Outside the nucleus |
| 5 | Discovery | E. Rutherford (1909) | James Chadwick (1932) | J.J. Thomson (1897) |
Conclusion
The discovery of subatomic particles like protons, neutrons, and electrons has helped develop an atom’s atomic structure. Because of this discovery, the modern periodic table has been developed and based on this development.
Many other concepts like chemical bonding by Kossel-Lewis, molecular orbital theory, valence shell electron repulsion theory, valence bond theory and a few of the complex chemical and physical properties can be explained using these particles.